[carousel_slide id=’256′]
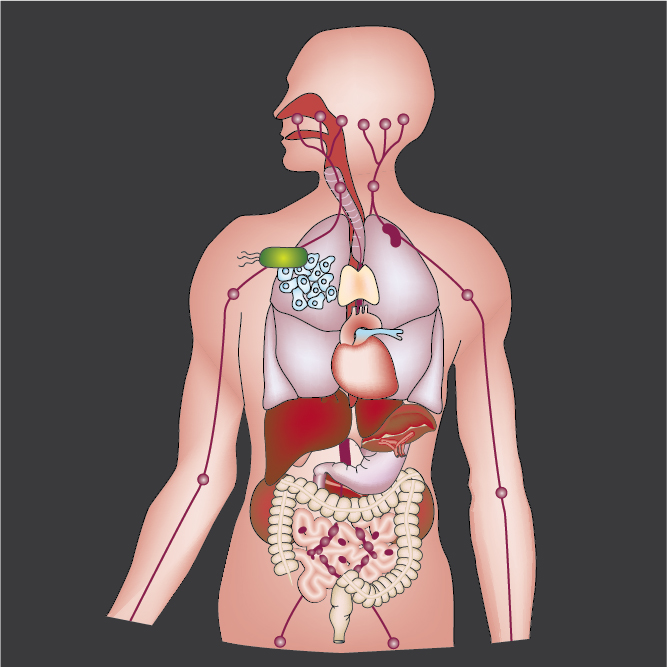
Bacterial Cancer Therapy
Therapeutic bacteria have unique properties that enable them to treat inoperable tumors and metastatic disease. These organisms are motile and actively penetrate tumor tissue. Therapeutic bacteria are nontoxic and specifically accumulate in solid tumors. This targeting enables delivery directly to malignant tissue, while sparing healthy organs. Inflammation, chemotaxis and growth are the dominant mechanisms that control tumor colonization. When in tumor tissue, bacteria use chemoreceptors to sense and migrate toward small molecules produced by cancer cells. Understanding and manipulating these mechanisms is the key to developing effective therapies that are suitable for human use and that eliminate drug-resistant tumors and metastases.
Intracellular Delivery of Protein Drugs
Critical cancer pathways often cannot be targeted because of limited efficiency crossing cell membranes. To address this challenge, we have created a system for intracellular delivery that utilizes the intrinsic cell invasion mechanisms of Salmonella. These engineered bacteria contain genetic circuits that (1) activate genetic regulators to drive invasion and (2) induce lysis to release protein drugs. Released proteins diffuse throughout the cellular cytoplasm and interact specifically with their therapeutic targets. The autonomous triggering of lysis after invasion makes the platform self-limiting and prevents drug release in healthy organs, greatly increase safety compared to other bacterial therapies. We have shown that the delivery of cytotoxic proteins with these engineered bacteria prevents the growth of solid tumors and metastases. Using bacteria to interrupt intracellular protein-protein interactions will enable targeting of disease-specific signaling pathways and will play a critical role in the next generation of biological therapies.
Bacterial Immunotherapy
Engineered bacterial therapies that target the tumor immune landscape offer a new class of cancer immunotherapy. We have engineered Salmonella to target tumors and serve as delivery vehicles for immunostimulatory molecules. These bacterial therapies induce both the innate and adaptive immune systems, and change the immune dynamics of the tumor microenvironment. As delivery vehicles, bacteria also have innate adjuvant properties that enhance immune responses. Harnessing a diverse set of mechanisms to alter the tumor-immune environment has the potential to generate many new and effective cancer therapies.
Synthetic Biology of Biomedical Tools
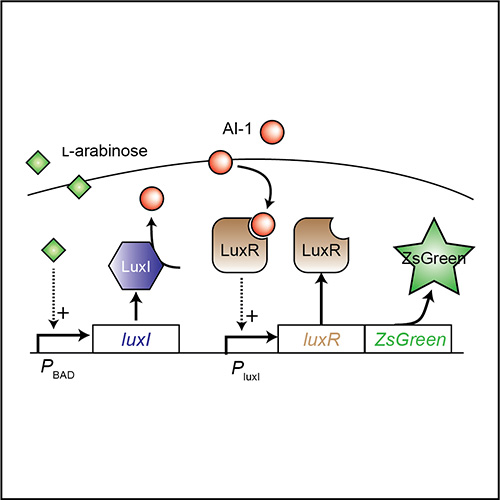
The microbial genome is highly plastic and malleable. Reconfiguration of biological components enables bottom-up design of novel therapeutic tools. Using genetic tools, we have created bacteria that deliver a wide range of therapeutic molecules, including microbial toxins, epigenetic regulators and immune modulators. We have created bacteria that detect very small tumors and are one hundred thousand times more sensitive than current tomography techniques. It is critical to control the timing and location of gene expression to maximize the efficacy and safety of bacterial delivery. To achieve this, we have created density-sensitive triggers that only permit protein production within tumors after bacterial colonization. We have also created bacterial-communication circuits that increase protein production and the sensitivity to induction signals. By taking cues from living systems, we are using rational design to create a large array of biomedical tools to treat human disease.
Computational Tumor Modeling
Understanding the interactions between intracellular metabolism, cell growth, and molecular transport is necessary to predict the efficacy of cancer therapy. Limited diffusion of nutrients and oxygen creates region of hypoxia and hypoglycemia. These poorly oxygenated regions are unresponsive to most cancer therapies. In average tumors, oxygen transport has a greater effect than glucose transport on the distribution of quiescent cells. Counterintuitively, tumors with less proliferating cells are more responsive to chemotherapeutics than tumors with more proliferating cells because of cell re-population. Based on MRI images from breast cancer patients, increased transvascular transport is associated with tumor aggressiveness and greater transport heterogeneity causes poor drug responsiveness. Nutrient gradients, intracellular metabolism, and cell growth are tumor mechanisms should be taken into account when developing cancer therapies. Being able to predict drug responsiveness would be invaluable for patients and oncologists when developing treatment regimens.
Drug Delivery
Molecular transport can define whether a molecular therapy will be effective. The interactions between transport mechanisms are complex and non-obvious. To address this critical problem, we have created in vitro devices that mimic the tissue surrounding blood vessels in tumors and enable measurement of diffusion, cell binding, and therapeutic outcome. Quantitative measurement in these devices has shown that cell attachment is critical for some cancer drugs, an effect that is masked when the drugs are encapsulated in stealth liposomes. When cancer drugs are bound to nanoparticles, surface charge and cellular uptake control the rate of cell uptake and delivery. Quantifying molecular transport in biological systems by integrating computational models, microfluidic devices, and animal models, enables the design effective therapeutics and drug-delivery systems.