The past two weeks have kept me out the lab thanks to a cold, a visiting scientist in my lab for a week, and unusually aggressive school work. But the week before, experiments ruled. Big experiments, or at least experiments on a big apparatus. By big, I mean the size of Rhode Island. Well, ok, not quite. In my life as a University scientist, before two weeks ago, the largest instrument I had been in the same room with was an electron microscope, a piece of gear that even with the associated chillers and power supplies could fit in a bathroom. Now, imagine 1,000 electron microscopes, and you will have some idea of the scale of the National Synchrotron Light Source-II (NSLS-II).

A synchrotron is a ring of electrons moving near the speed of light. Because they are charged and because of design features of ring (taking advantage of a bunch of physics that I do not really understand), the circulating electrons emit photons, termed logically enough, synchrotron radiation. The electrons are accelerated to vast energies and thus the emitted radiation is bright, really bright. Laser beams are bright too but lasers emit photons at a single wavelength; synchrotron radiation is polychromatic, spanning the spectrum from gamma rays to radio waves. This is what makes synchrotrons so attractive – scientists can peel off a waveband of interest, leaving all the rest still zooming round the ring. This peeling-off happens at beam lines, spokes tangent to the ring and containing a pile of associated instrumentation needed to do something of interest with a beam of such-and-such a character.
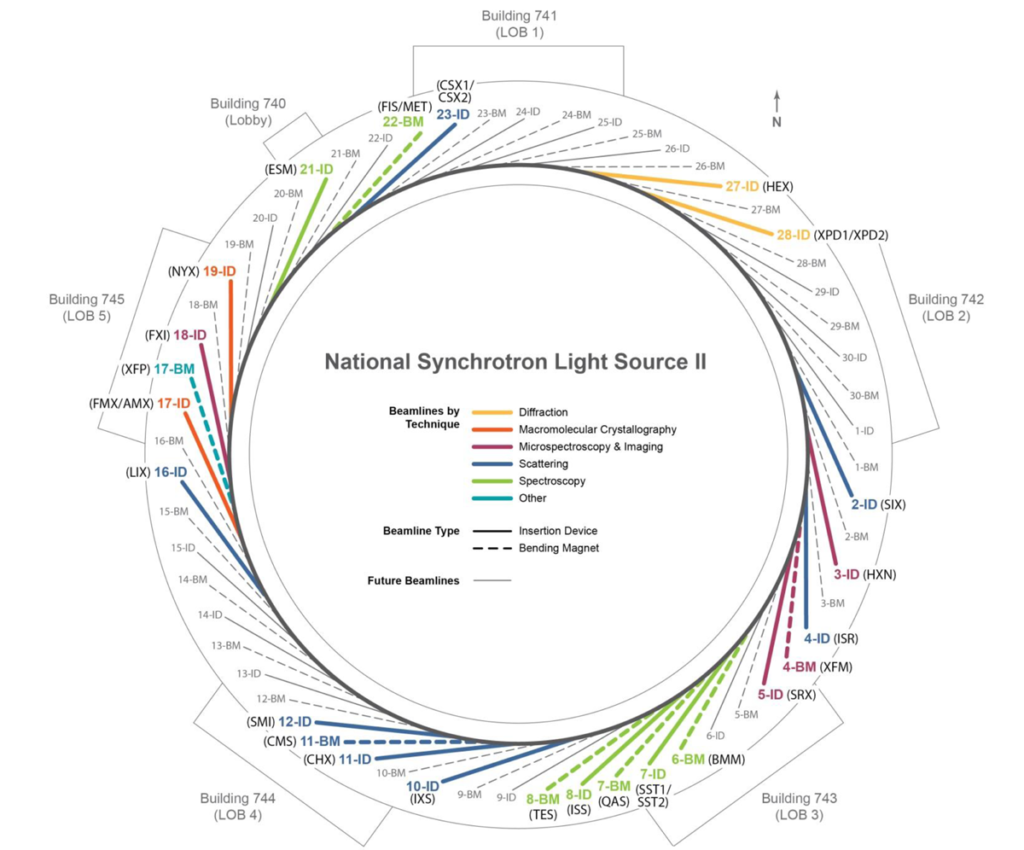
The NSLS-II, which opened about a year or two ago, is at Brookhaven National Laboratory (BNL) and run under the aegis of the US Department of Energy (DOE). Because it is a National Laboratory they would like nationals (and I hasten to add internationals too, whom they have been fairly and effectively screening since BNL opened without issue) to come have a go. If you have an idea of something you would like to light up with synchrotron radiation, fill out their handy application forms. Even more than a general national interest, it happens that, my research is funded by the DOE and that program likes to see its fundees using the National Labs. Always willing to try to make my program officer happy, right?
And I do have an idea for something to light up with synchrotron photons, specifically with X-rays—the growth zone of the root. X-rays for years have been used to study the structure of wood. This is because cellulose, a major component of wood, is partly crystalline and crystals scatter X-rays. Wood is packed with cellulose crystals, the material is, well, stable as houses, and so X-rays have provided invaluable information about how cellulose is organized, in wood. Now, I have long with worried about how cellulose is organized, but not in wood – rather in the growing region of the root. The growing root has thin cell walls, with only a little bit of cellulose. The cells themselves are full of water and cytoplasm, all conditions that defeat X-rays. Or so I thought until my friend Ingo Burgert and his colleagues published a paper where they use X-ray scattering to determine the orientation of cellulose in a seedling stem. A living stem.
To get similar information, I have been painstakingly cutting thin sections and imaging them with polarized-light microscopy, or just as painstakingly cutting slightly thicker sections and using scanning electron microscopy. Either way the sectioning is tedious and only small bits of the whole can be assessed. Apparently, X-rays are now able to the job with an intact and hydrated plant. Wow!
Ingo’s crew used a synchrotron in Germany but here I am living about a 2.5 h drive away from NSLS-II. I wound up getting a scientist, Lin Yang, on the phone, a person who coordinates the kind of X-ray technique I wanted to use (SAXS and WAXS, which sound like something out of Dr Seuss but mean small-angle and wide-angle X-ray scattering), and after convincing him that my idea was not entirely bonkers, and filling out the application for beam time, all of a sudden there I was with a day to play.
Right after class on Wednesday, I bundled some seedlings into the back of my car, along with a box of lab stuff that might prove handy, and drove down to the ferry at Bridgeport and then to BNL. Getting to take a ferry ride was definitely an added perk on what was a crispy sunny day. At the BNL gate, I was given a temporary parking pass and at the admin building, a photo i.d., key card, and a parking pass good for a year. That was more optimistic than I felt about the project.
The ring of NSLS II is so big that it has five buildings around the periphery controlling access (see figure 1). Each one of these buildings is about the size of a small airport terminal. I found the one I was supposed to report to and checked in there too, getting my dosimeter, which I was required to wear any time I was on site. Then I used my newly minted key card to enter the area with the beam lines. Think giant mill-building or hangar that curves gently out of sight in each direction. The walkway encircling the facility is so large that there are trikes to share placed strategically here and there. I had to find my beam line, one of about two dozen that are active (another ten or so are under construction). I walked clockwise, past mobile-home sized metal-clad labs with occasional open hutches giving a glimpse of stainless tubes that would gladden the heart of any Hollywood set designer, past small work shops and past open areas of the floor used for storage.

At last, I found beam line 16-ID, aka ‘life sciences X-ray scattering, LiX’ and met Shirish and Vini, staff who work with Lin. Although Vini ought by rights to have had a deep Long Island accent, he hails from farther away. They showed me the ‘wet lab’ where I would be allowed to manipulate the plants, getting them to a state where they could be examined by the beam. This room was about a mile from LiX, ok, not that far but a long walk around the ring all the same. We put the plants under the light on a bench and unpacked the box, but nothing was to be done until tomorrow.
The next day, after a ten minute commute on the Long Island Expressway, I showed up for work. Because the synchrotron had been off for the last few days for maintenance, the first order of business was getting the beam back to a useful condition. X-rays are a lot harder to focus than visible light (burning holes in things is just one of the problems) and the lenses are made of beryllium. Hearing Lin explain this, I might as well have been getting a lesson from Scottie on warp drive. While they were sprucing up the beam, I went off to do the same to my plants.
The problem is background. In the best case, the root would be suspended in air. But my arabidopsis roots are so thin (about 0.1 mm in diameter) that they would dry out in a trice if suspended in air. Even worse, the WAXS detectors are only in two positions and to see the signal we expected, the root would best be placed horizontally. Well, these roots are so thin that this would be impossible with a root in suspended freely air. Try placing a piece of cooked spaghetti horizontally to get an idea of the problem. Wilt. Next best to air is mica, a thin shelf of mica that can support the root but not absorb or scatter too many X-rays. Shirish showed me how to take large hunks of mica and peel them to get nice thin bits. But the other problem is water—how to keep the roots hydrated. My idea was to place the root on an agar substrate, indeed I brought with me a bottle of nutrient-agar medium just for this purpose.
Finally there is a metal holder, about 2 inches by 1 inch, with 8 holes drilled through. The sample is placed on the holder, with the region of interest placed over a hole, and the holder snaps onto the rig for exact positioning in the beam. I put a thin slab of mica on the holder, held down with good old double-sticky tape, and then dropped on a few drops of molten agar, allowing it to run close to but not over the hole. Once it had set, I put the root tip on the mica right over the hole and laid the rest of the root and the shoot on the agar. The idea being that the root tip would stay hydrated by virtue of the agar – water reservoir.
We watched the X-ray beam can cut through the root, severing it completely. We learned that the roots dry out despite the proximity of water, though it might be slow enough to get a valid ‘wet’ run with care. But despite all that, we did get SAXS scattering patterns, indicative of cellulose (Fig. 4). The only problem is that the cellulose seems to be going the ‘wrong’ way.
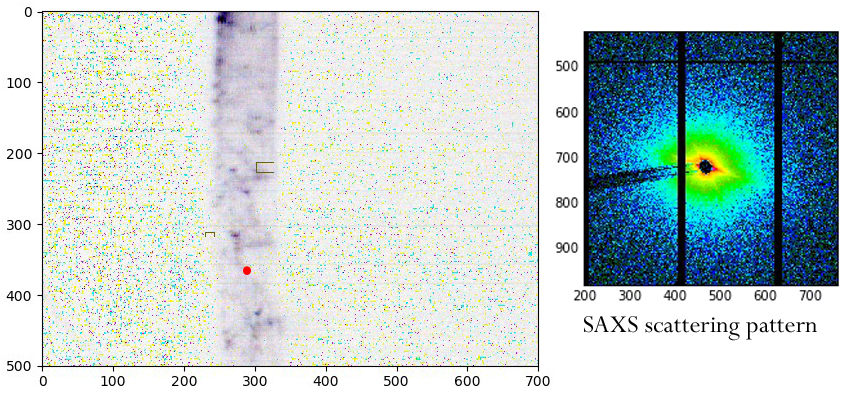
This was not a raging success. The small roots of arabidopsis were difficult to handle and I consider it likely that the signals we saw were dominated by drying artifacts. It might be possible to image directly through the agar, perhaps on a wire loop. Or we might be able to use a thicker root, one that would stay wet better, and inter alia have more cellulose in the beam. I was heartened to learn that Lin thinks so too and I have been invited to apply for more time!
Prof. Baskin, I am only 1 hr away from BNL. Please let me know when you come back for your next experiment. We shall have a dinner or something to catch up.
Ben
Ben – I’d love to! But only if you call me “Tobias”!
Dear Tobias:
One of my colleagues spent 8 years in BNL and has extensive experiences with protein crystallography. Your dehydration issue is similar to what they faced in protein crystallography. The solution is to use preservatives such as PEG to preserve your roots and freeze them in LN. They have the whole setup in protein crystallography division.
Hope this helps.
Regards
Ben